CRISPR 2.0: Editing Genes in Real Time
In 2012, the discovery of CRISPR-Cas9 revolutionized genetics. Scientists could now cut and edit DNA with unprecedented precision. But the revolution didn’t stop there.
Welcome to CRISPR 2.0—the next leap in gene editing, where researchers are pushing the boundaries of biology to edit genes in real time, inside living organisms, with more accuracy and fewer side effects than ever before.
This isn’t just about fixing genes in a lab. It’s about treating diseases as they happen. And it’s happening faster than you think.
🧬 From CRISPR to CRISPR 2.0: What Changed?
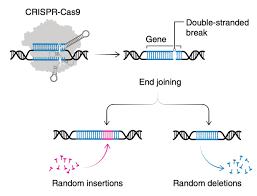
CRISPR-Cas9 allowed us to cut DNA at specific points and introduce changes. But it had limitations:
-
Off-target effects: Cutting the wrong DNA segment could be dangerous.
-
Limited control: Once activated, the editing tool would cut indiscriminately.
-
One-time edits: Changes were permanent and hard to reverse.
CRISPR 2.0 addresses these challenges with smarter, safer, and more flexible systems:
🔹 Base Editing
Instead of cutting, base editors swap one DNA letter for another, like fixing a typo in a word. No breaks, no damage—just precision.
🔹 Prime Editing
Prime editors act like a word processor for DNA, allowing scientists to search, replace, or delete entire sequences without breaking the strand.
🔹 Real-Time, In-Body Editing
With advances in delivery systems (like lipid nanoparticles and viral vectors), researchers can now edit genes inside living bodies—not just in a petri dish.
🧪 Real-Time Treatments in Action
🧠 Neurological Disorders
Gene editing tools are being delivered directly into the brain to treat conditions like Huntington’s disease and ALS. Scientists can now adjust faulty genes while the disease is progressing, offering new hope for conditions once deemed untreatable.
👁️ Blindness
In clinical trials, patients with a rare form of inherited blindness have received CRISPR treatments via injections directly into their eyes. The therapy aims to restore vision by correcting faulty DNA on the spot.
🩸 Blood Disorders
Diseases like sickle cell anemia are being treated by editing bone marrow stem cells, allowing patients to produce healthy blood cells—sometimes with just a single treatment.
🛡️ Safety and Ethics
With great power comes great responsibility. CRISPR 2.0 brings us closer to real-time genetic correction, but it also raises new questions:
-
What if we edit the wrong gene in a living person?
-
Should we allow edits that enhance traits rather than treat disease?
-
How do we regulate therapies that change human DNA permanently?
Regulatory bodies like the FDA and international bioethics councils are working to keep pace, but the science is moving fast.
🌍 Global Implications
CRISPR 2.0 isn’t just about medical miracles. It’s also being explored for:
-
Climate-resilient crops
-
Eradicating disease-carrying insects
-
Protecting endangered species through gene rescue
In real time, we may soon be editing life on Earth to adapt to the future.
🔮 What’s Next?
The next phase of CRISPR innovation could include:
-
Real-time feedback loops, where gene edits adjust dynamically as the body responds.
-
Tissue-specific editing, targeting only certain organs or cells.
-
Reversible edits, allowing for temporary genetic changes.
The line between biology and technology is blurring—and CRISPR is at the center of it all.
🧠 Final Thought
CRISPR 2.0 is turning science fiction into reality. We’re no longer just studying DNA—we’re rewriting it, live, inside the body.
This technology may one day cure genetic diseases, extend human life, and reshape how we understand evolution itself.
The future isn’t just coming—it’s being edited.